Referral Resources
Mammography alone is not enough
Mammography remains the primary screening method for the detection of breast cancer, yet it is now recognized by the medical community and federal government, that mammography alone is not sufficient for women with dense breasts.
Dense Breast Imaging Facts:



Sources:
• Berg, W.A. Breast MRI for “the Masses”. Eur Radiol 32, 4034–4035 (2022);
• Densebreast-info, inc.. DenseBreast. (2023, May 18). Source.

Why SoftVue™ for Dense Breast Screening?
While mammography is highly effective for women with BIRADS a or b breast density, mammography misses cancer in women with BIRADS c or d breast density as both cancer and fibroglandular tissue appear white on mammogram.
SoftVue was developed specifically to address the unmet need for effective dense breast cancer screening.
When combined with mammography:


BIRADS 3 threshold

SoftVue™ Benefits
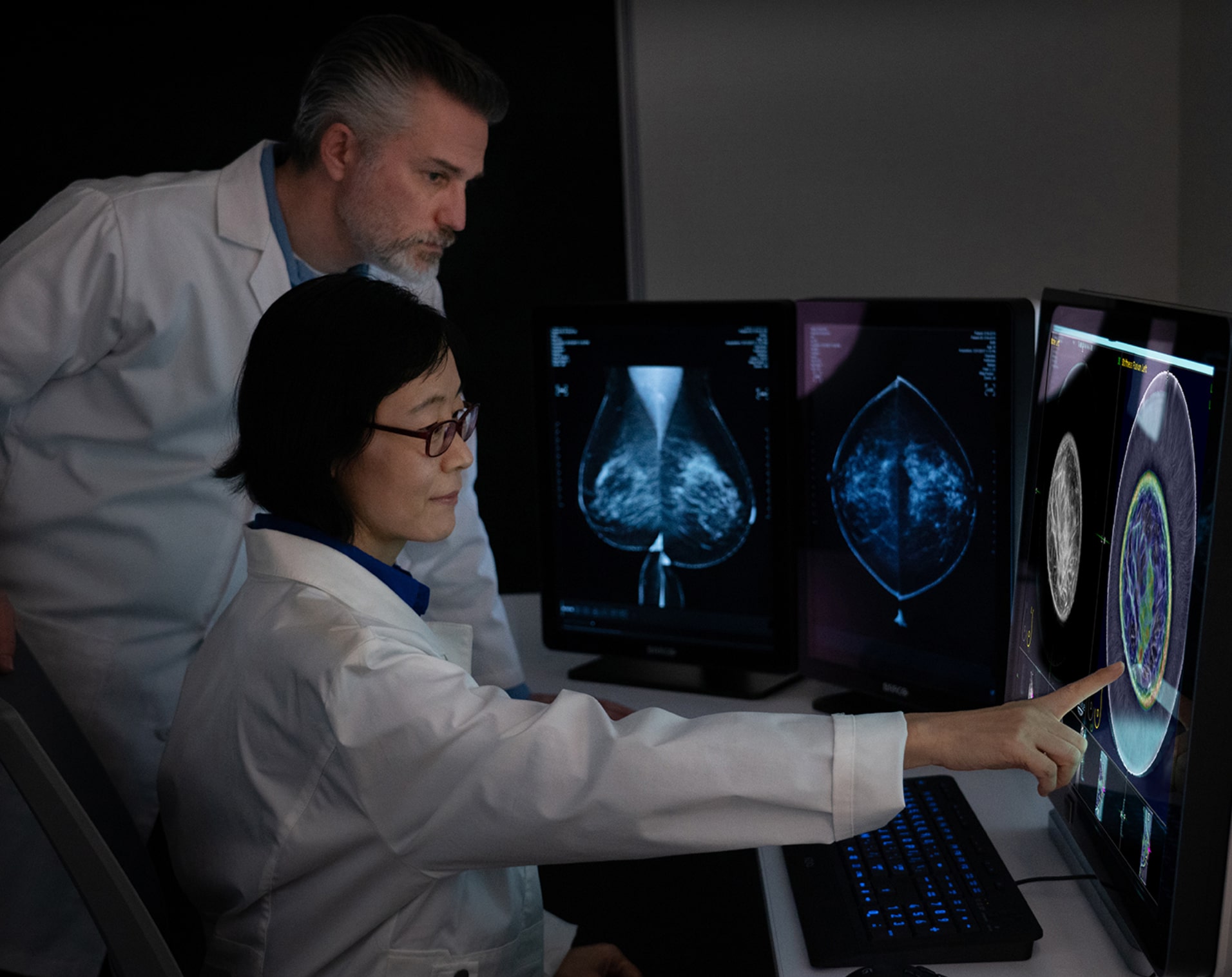
Central to SoftVue’s innovation is the circular transducer and proprietary algorithms that power its TriAD triple acoustic detection technology, an ultrafast 360-degree electronic sequencing collecting reflection, speed of sound, and attenuation sound signals, presenting not only breast tissue structure but tissue characterization as well.
Increases in specificity and sensitivity are achieved through this proprietary technology. The three sound signals collected during image acquisition are reconstructed into 4 image sequences presented for SoftVue interpretation in combination with mammography. These 4 sequences provide not only better cancer detection, but also better characterization of masses at the point of screening.
SoftVue offers additional patient benefits including:
- No compression
- No radiation
- No contrast injection
- FDA approval for use on same day as mammography
Women with dense breasts have a
4-6 times greater risk
of developing breast cancer compared to women with fattier breasts
How to Refer Your Patients for SoftVue™
SoftVue is covered under existing insurance codes; CPT 76641. If you have patients that have dense breasts, when writing the order for annual mammogram, simply add whole breast ultrasound in addition to 2D and/or 3D mammogram.
Sample language that can be used:
“2D, digital breast tomosynthesis/3D mammogram; screening ultrasound due to dense breast tissue*”
During image interpretation, the radiologist can reference ICD-10 code R92.2 or R92.3, this is typically used as the indication for supplemental imaging for women with dense breasts for billing purposes.
*Information provided by DenseBreast-info.org (2023).
FREE CME and CE
Evidence regarding the limited effectiveness of mammography for dense breast screening has been widely published in peer reviewed journals and publicized in the media. This raised awareness led to the FDA’s consideration and, in March 2023, of updated MQSA guidelines requiring as of September 2024:
- All mammography facilities in the U.S. to notify women if their breasts are “dense” or “not dense.” And, a written report to referring providers that includes assessment of the specific breast density category (fatty, scattered, heterogeneously dense or extremely dense).
- Patient notification to include “…dense tissue makes it harder to find cancer on a mammogram and also raises the risk of developing breast cancer.”
- Direction to “talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”
These new guidelines increase the need for physicians to understand the impact of breast density on patients’ cancer risk and how supplemental imaging can benefit women with dense breasts.
If you need educational tools on breast density, including a free CME/CE course, Dense Breasts and Supplemental Screening, please visit DenseBreast-info.org.
Visit DenseBreast-info
